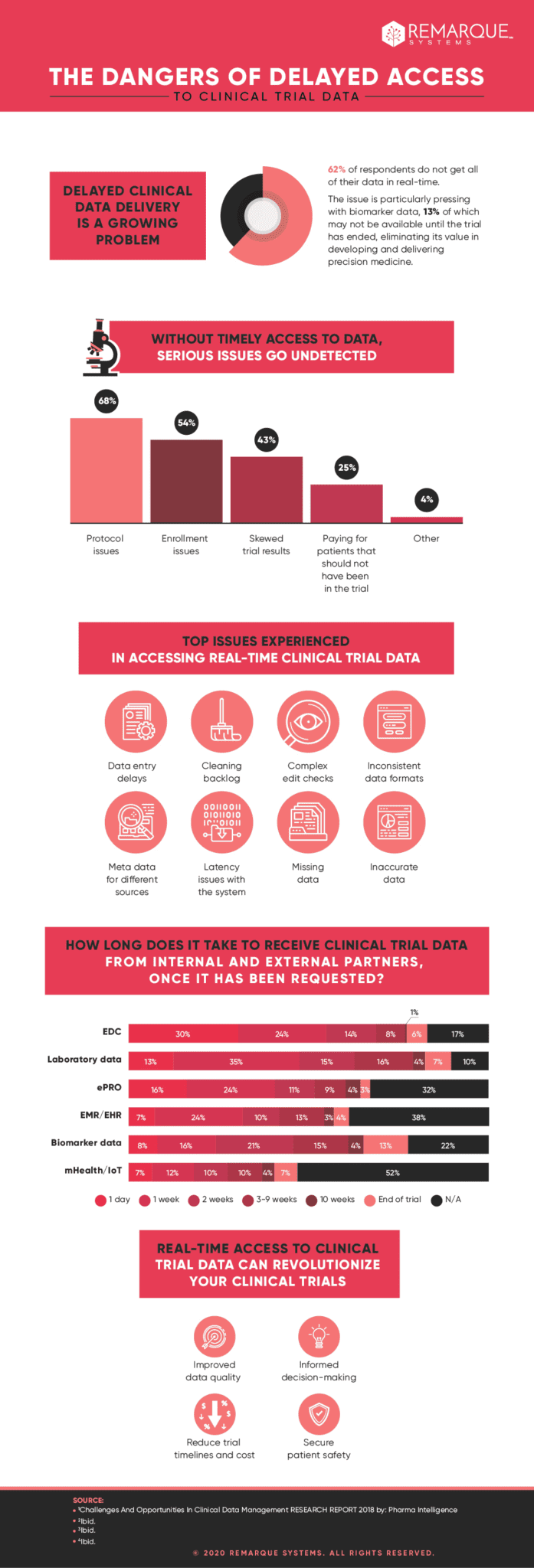
Delayed clinical trial data delivery is a growing problem for pharmaceutical companies and one that can have catastrophic consequences for the development of their promising pipeline candidates.
When data delivery lags behind the pace of a clinical study it means that data entry will be delayed – especially if the data arrives in multiple formats or from a number of different sources that then need to be reconciled.
The upshot of these issues can be a series of serious setbacks, as clearing redundant data becomes different and programming complex edit checks becomes next to impossible.
As illustrated in a new infographic from Remarque Systems (scroll down to see it), delays with the delivery of clinical trial data are a growing problem across the pharmaceutical industry, with important ramifications for studies in a number of areas, including personalized medicine.
The clinical trial optimization company says that while delays in data transfer from external and internal partners have long been an expected inconvenience in the course of a clinical trial, the situation is quickly becoming untenable as the amount and variety of trial-related data multiples.
Where researches are unable to view or verify data they face errors, delays, inefficiencies and costs that should be entirely avoidable, Remarque Systems says.
A lack of real-time access to all of the available clinical trial data also means that clearing redundant data becomes difficult, and programming complex edit checks becomes impossible.
When it comes to accessing their clinical data, the majority of respondents in a 2018 Pharma Intelligence survey said they did not have real-time access to their data, which can cause problems that can go undetected until it is too late.
The pharmaceutical industry faces continual pressure to optimize clinical trials, maximize returns and minimize risk. Better data quality can improve decision-making, making companies more informed, shrinking trial timelines and costs, and securing patient safety.